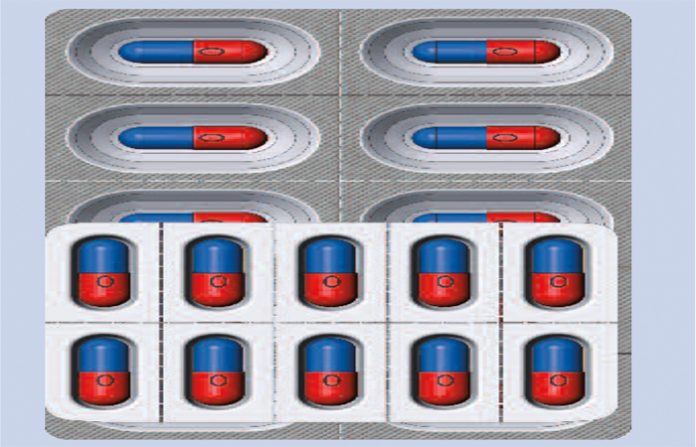
On 20 August 2015, Honeywell launched Safyr, a high-moisture barrier thermoformable film designed specifically to meet the requirements of India’s domestic pharmaceutical packaging market. Safyr, pronounced sapphire, is a CTFE-based polymer film with excellent moisture barrier properties that is designed specifically to meet local packaging requirements while remaining cost-effective for the domestic Indian market. Safyr affords manufacturers with design flexibility, does not yellow over time, has a longer storage life compared to alternatives and requires no pre-conditioning prior to operational use, all while providing superior moisture barrier.
“As a world leader in pharmaceutical packaging materials, Honeywell is working to develop industry leading packaging solutions that can deliver increased competitive advantage. Honeywell optimized Safyr to meet regulatory requirements specific to the domestic pharmaceutical push through the packaging market in India,” said Scott Gaddis, global business leader for healthcare products in Honeywell’s Packaging and Composites business. “Safyr is standardized with one width, one length and one thickness, which helps in process and cost optimization.”
Safyr is available in a 2 mil (51μ) thickness, and provides an alternative option to cold form foil or foil strip packs as the package format to pass stability. By utilizing a thermoforming platform, pharmaceutical companies can decrease their pack size versus foil formats and increase pack density, while improving patient acceptance.
With Safyr films, packaging capacity can be increased with the same line speed, allowing significant reduction in capital expenditure for new or additional blister lines – thus increasing the manufacturer’s overall equipment efficiency (OEE). The reduced pack size means fewer raw materials, including primary packaging material, lid stock and secondary packaging, leading to reduced total cost of goods.










