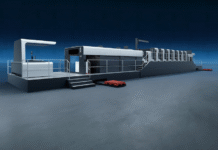
In 2017, the Sidel Aseptic Combi Predis blow fill seal filler was validated by the American Food and Drug Administration (FDA) for low acid products sold in the US – an industry first. With more than 180 references at major beverage and dairy companies since the launch of this solution in early 2000, the repeated orders recorded by this technology underline its success. According to Sidel, since the first FDA approval, many beverage and dairy players, located in the US, Asia, and South America, have trusted the company’s dry preform decontamination technology to develop their business in the US. This customer success reflects the global achievements collected by Predis technology since its launch — it has decontaminated more than 60 billion bottles while saving over 10 billion liters of water and 60,000 tons of PET, Sidel said. The Aseptic Combi Predis differs from former aseptic bottling technologies because the PET package sterilization already occurs at the preform stage rather than during the bottling phase. The solution marks an essential step towards sustainable production because it…
Choose your subscription to read more
Trial
- ₹ 0 for 4 weeks*
PSA Plan 1
- ₹1,500.00 for 1 year*
PSA Plan 2
- ₹2000 for Year*
PSA Plan 3
- ₹3,600.00 for 2 year*